
Chemistry, 19.02.2020 22:59 Picklehead1166
Sodium metal (atomic weight 22.99 g/cm^3) adopts a body-centered cubic structure with a density of 0.97 g/cm^3. (a) Use this information and Avogrado's number (Na=6.022x10^23) to estimate the atomic radius of sodium. (b) If it didn't react so vigorously, sodium could float on water. Use the answer from part (a) to estimate the density of Na if its structure were that of a cubic close-packed metal. Would it still float on the water?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1


Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 22.06.2019 22:00
8) warming your hands by a fire is an example if which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 1
You know the right answer?
Sodium metal (atomic weight 22.99 g/cm^3) adopts a body-centered cubic structure with a density of 0...
Questions

Mathematics, 23.09.2021 15:20

World Languages, 23.09.2021 15:20

English, 23.09.2021 15:20

Social Studies, 23.09.2021 15:20


English, 23.09.2021 15:20


Social Studies, 23.09.2021 15:20

English, 23.09.2021 15:20

Mathematics, 23.09.2021 15:20

Mathematics, 23.09.2021 15:20

Computers and Technology, 23.09.2021 15:20


Mathematics, 23.09.2021 15:20

Mathematics, 23.09.2021 15:20

Mathematics, 23.09.2021 15:20

Biology, 23.09.2021 15:20

Chemistry, 23.09.2021 15:20

Business, 23.09.2021 15:20

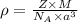
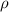 = density =
= density = 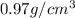
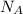 = Avogadro's number =
= Avogadro's number = 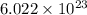
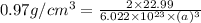
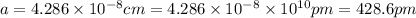
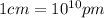
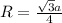
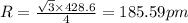
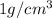
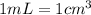 )
)

