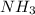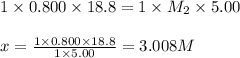Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2

You know the right answer?
If 18.8 mLmL of 0.800 M HClM HCl solution are needed to neutralize 5.00 mLmL of a household ammonia...
Questions

Health, 14.09.2020 07:01

Mathematics, 14.09.2020 07:01

Mathematics, 14.09.2020 07:01

History, 14.09.2020 07:01

Geography, 14.09.2020 07:01

Mathematics, 14.09.2020 07:01

Mathematics, 14.09.2020 07:01

English, 14.09.2020 07:01

Mathematics, 14.09.2020 07:01

Mathematics, 14.09.2020 07:01

Physics, 14.09.2020 07:01

Mathematics, 14.09.2020 07:01

Health, 14.09.2020 07:01

Mathematics, 14.09.2020 07:01

Mathematics, 14.09.2020 07:01

History, 14.09.2020 07:01

Mathematics, 14.09.2020 07:01

History, 14.09.2020 07:01

English, 14.09.2020 07:01

Health, 14.09.2020 07:01

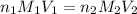
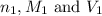 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 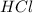
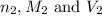 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is