Chemistry, 20.02.2020 00:14 cheerleaderautumnche
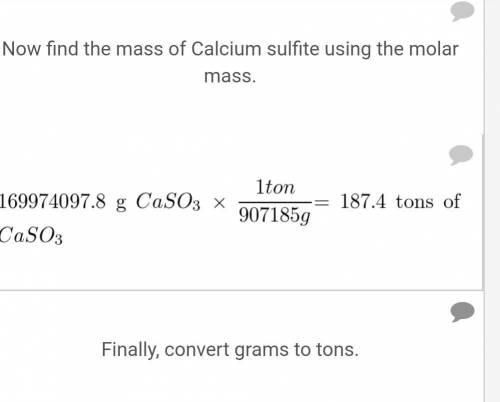
A particular coal contains 2.5% sulfur by mass. When this coal is burned at a power plant, the sulfur is converted into sulfur dioxide gas, which is a pollutant. To reduce sulfur dioxide emissions, calcium oxide (lime) is used. The sulfur dioxide reacts with calcium oxide to form solid calcium sulfite. If the coal is burned in a power plant that uses 2000.0 tons of coal per day, what mass of calcium oxide is required daily to eliminate the sulfur dioxide?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3
You know the right answer?
A particular coal contains 2.5% sulfur by mass. When this coal is burned at a power plant, the sulfu...
Questions


Business, 24.09.2019 12:30


Chemistry, 24.09.2019 12:30

Mathematics, 24.09.2019 12:30


Biology, 24.09.2019 12:30


History, 24.09.2019 12:30





History, 24.09.2019 12:30

English, 24.09.2019 12:30

History, 24.09.2019 12:30


Mathematics, 24.09.2019 12:30

World Languages, 24.09.2019 12:30