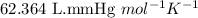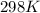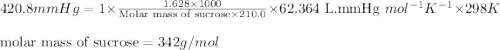Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
A solution prepared by dissolving 1.628 g of sucrose, a nonelectrolyte, in enough water to produce 2...
Questions

Biology, 28.05.2021 08:10

Mathematics, 28.05.2021 08:10



Mathematics, 28.05.2021 08:20

Biology, 28.05.2021 08:20

Mathematics, 28.05.2021 08:20

Health, 28.05.2021 08:20


Mathematics, 28.05.2021 08:20





Mathematics, 28.05.2021 08:20


Physics, 28.05.2021 08:20

Biology, 28.05.2021 08:20


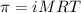
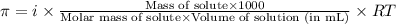
 = osmotic pressure of the solution = 420.8 mmHg
= osmotic pressure of the solution = 420.8 mmHg