
Chemistry, 20.02.2020 01:37 blackops3318
A solution in which the molarity of the hydroxide ion is larger than the molarity of the hydronium ion is termed 1. amphiprotic. 2. electrolyte. 3. basic. 4. neutral. 5. acidic.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2
You know the right answer?
A solution in which the molarity of the hydroxide ion is larger than the molarity of the hydronium i...
Questions

Mathematics, 07.07.2021 22:40

Mathematics, 07.07.2021 22:40

Mathematics, 07.07.2021 22:40


Chemistry, 07.07.2021 22:40


Mathematics, 07.07.2021 22:40



Social Studies, 07.07.2021 22:40


English, 07.07.2021 22:40


English, 07.07.2021 22:40

Mathematics, 07.07.2021 22:40

Mathematics, 07.07.2021 22:40

Mathematics, 07.07.2021 22:40

Mathematics, 07.07.2021 22:40


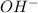 as compared to
as compared to 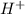 .
.

