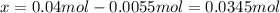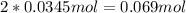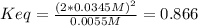Chemistry, 20.02.2020 01:58 brendanhein1
Dinitrogentetraoxide partially decomposes into nitrogen dioxide. A 1.00-L flask is charged with 0.0400 mol of N2O4. At equilibrium at 373 K, 0.0055 mol of N2O4 remains. Keq for this reaction is .

Answers: 1


Another question on Chemistry




Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1
You know the right answer?
Dinitrogentetraoxide partially decomposes into nitrogen dioxide. A 1.00-L flask is charged with 0.04...
Questions

Chemistry, 01.12.2020 03:00


History, 01.12.2020 03:00

Mathematics, 01.12.2020 03:00


Biology, 01.12.2020 03:00


Mathematics, 01.12.2020 03:00



Computers and Technology, 01.12.2020 03:00


History, 01.12.2020 03:00

Advanced Placement (AP), 01.12.2020 03:00




History, 01.12.2020 03:00


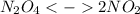
 ) was:
) was: