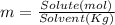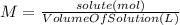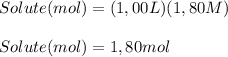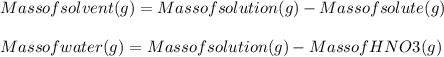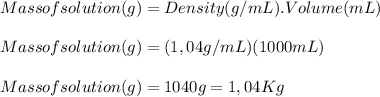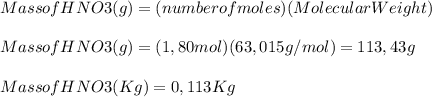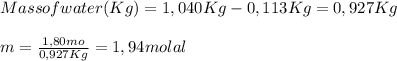Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3


Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
You know the right answer?
A 1.80 M aqueous solution of nitric acid has a denstiy of 1.04 g/ml. What is the molality of the sol...
Questions



Mathematics, 19.11.2019 03:31

Mathematics, 19.11.2019 03:31


Mathematics, 19.11.2019 03:31



Mathematics, 19.11.2019 03:31


Mathematics, 19.11.2019 03:31

History, 19.11.2019 03:31


Mathematics, 19.11.2019 03:31






English, 19.11.2019 03:31