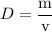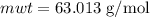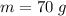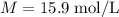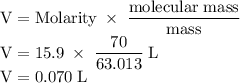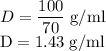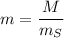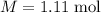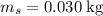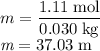Chemistry, 20.02.2020 03:38 anabelleacunamu
The concentration of commercially available concentrated nitric acid is 70.0 percent by mass, or 15.9 M. Calculate the density and molality of the solution.\

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3
You know the right answer?
The concentration of commercially available concentrated nitric acid is 70.0 percent by mass, or 15....
Questions


Mathematics, 15.01.2021 23:50

Mathematics, 15.01.2021 23:50

Mathematics, 15.01.2021 23:50

Mathematics, 15.01.2021 23:50


Mathematics, 15.01.2021 23:50



Mathematics, 15.01.2021 23:50



Biology, 15.01.2021 23:50

English, 15.01.2021 23:50

Social Studies, 15.01.2021 23:50

Mathematics, 15.01.2021 23:50

Mathematics, 15.01.2021 23:50

Mathematics, 15.01.2021 23:50

Arts, 15.01.2021 23:50

Computers and Technology, 15.01.2021 23:50