Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1


Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2
You know the right answer?
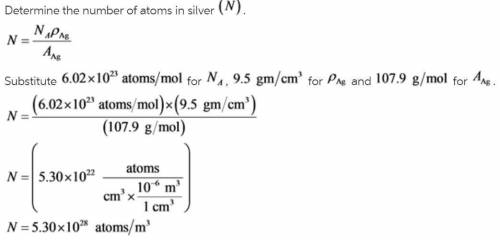
Alculate the energy for vacancy formation in silver, given that the equilibrium number of vacancies...
Questions

Mathematics, 03.12.2020 04:50

Mathematics, 03.12.2020 04:50


Mathematics, 03.12.2020 04:50


Mathematics, 03.12.2020 04:50


Mathematics, 03.12.2020 04:50


Arts, 03.12.2020 04:50


Mathematics, 03.12.2020 04:50

English, 03.12.2020 04:50


Mathematics, 03.12.2020 04:50



Chemistry, 03.12.2020 04:50

Mathematics, 03.12.2020 04:50