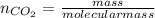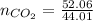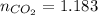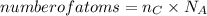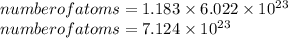Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 23.06.2019 08:00
Which term means two or more atoms that share electrons in a chemical bond? a. hydrogen bond b. moleculec. ionic bondd. element amd you
Answers: 3

Chemistry, 23.06.2019 10:30
The element chlorine has two stable isotopes, chlorine-35 with a mass of 34.97 amu and chlorine-37 with a mass of 36.95 amu. from the atomic weight of cl = 35.45 one can conclude that:
Answers: 2

Chemistry, 23.06.2019 11:30
The density of e85 fuel is 0.801 g/ml. what is the mass of 1.00 gallon of the fuel? (1 gal. = 3.785 l)
Answers: 3
You know the right answer?
How many carbon atoms are there in 52.06 g of carbon dioxide, CO2? The molar mass of CO2 is 44.01 g/...
Questions

Mathematics, 19.11.2019 19:31

English, 19.11.2019 19:31

History, 19.11.2019 19:31

Chemistry, 19.11.2019 19:31

Social Studies, 19.11.2019 19:31

Biology, 19.11.2019 19:31


Health, 19.11.2019 19:31

History, 19.11.2019 19:31

Social Studies, 19.11.2019 19:31

History, 19.11.2019 19:31


Health, 19.11.2019 19:31




Physics, 19.11.2019 19:31


History, 19.11.2019 19:31