Chemistry, 20.02.2020 09:40 oofoofoof1
The decomposition of NO2(g) occurs by the following bimolecular elementary reaction. 2NO2(g) → 2NO(g) + O2(g) The rate constant at 273 K is 2.3 x 10-12 L mol-1 s-1, and the activation energy is 111 kJ/mol. How long will it take (in s) for the concentration of NO2(g) to decrease from an initial partial pressure of 2.80 atm to 1.90 atm at 479 K? Assume ideal gas behavior.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3


Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
The decomposition of NO2(g) occurs by the following bimolecular elementary reaction. 2NO2(g) → 2NO(g...
Questions


Mathematics, 12.03.2020 22:25

Mathematics, 12.03.2020 22:25

English, 12.03.2020 22:25

English, 12.03.2020 22:25

Mathematics, 12.03.2020 22:25




Geography, 12.03.2020 22:26

Biology, 12.03.2020 22:26

Chemistry, 12.03.2020 22:26


Physics, 12.03.2020 22:26

Biology, 12.03.2020 22:26





![\ln(\frac{K_{479K}}{K_{273K}})=\frac{E_a}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0517/3905/b7df8.png)
 = equilibrium constant at 479 K = ?
= equilibrium constant at 479 K = ?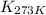 = equilibrium constant at 273 K =
= equilibrium constant at 273 K = 
 = Activation energy = 111 kJ/mol = 111000 J/mol (Conversion factor: 1 kJ = 1000 J)
= Activation energy = 111 kJ/mol = 111000 J/mol (Conversion factor: 1 kJ = 1000 J) = initial temperature = 273 K
= initial temperature = 273 K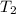 = final temperature = 479 K
= final temperature = 479 K![\ln(\frac{K_{479K}}{2.3\times 10^{-12}})=\frac{111000J}{8.314J/mol.K}[\frac{1}{273}-\frac{1}{479}]\\\\K_{479K}=3.132\times 10^{-3}L.mol^{-1}s^{-1}](/tpl/images/0517/3905/9840b.png)
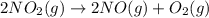

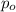 = initial partial pressure = 2.80 atm
= initial partial pressure = 2.80 atm