
Chemistry, 20.02.2020 07:00 kendasinjab
Consider a biochemical reaction that is taking place in a 0.1 M buffer. The initial pH is 7.4, and the pKa of the buffer is 7.2. If, in a final reaction volume of 1.0 mL, 10 micromol of protons are generated, what would be the final pH of the solution?

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 05:00
Which of the following describes qualitative data? a) recording the temperature of a solid as it is warmed. b) noting the color of a solution as it is heated. c) measuring the volume of an object by water displacement. d) taking the mass of an object using a balance.
Answers: 2

Chemistry, 23.06.2019 07:00
Achemist who studies water samples did a demonstration of how to test for lead in water. she added a clear solution of potassium iodide to a clear solution of lead nitrate. then a yellow swirling solid formed in the liquid. what is most likely true about the yellow solid?
Answers: 3

Chemistry, 23.06.2019 08:30
According to the passage, which of these is true about gray water systems? a) gray water systems use plants that require less water. eliminate b) gray water systems require the use of less fossil fuels. c) gray water systems reduce the amount of fresh water used. d) gray water systems reduce the amount water used by shower heads.
Answers: 1
You know the right answer?
Consider a biochemical reaction that is taking place in a 0.1 M buffer. The initial pH is 7.4, and t...
Questions





Spanish, 21.03.2021 01:00



Mathematics, 21.03.2021 01:00




Mathematics, 21.03.2021 01:00

Mathematics, 21.03.2021 01:00

Advanced Placement (AP), 21.03.2021 01:00

Geography, 21.03.2021 01:00




Mathematics, 21.03.2021 01:00

History, 21.03.2021 01:00

![pH= pKa +log\frac{[A-]}{[HA]}](/tpl/images/0517/0808/e9fef.png)
![7,4=7,2 + log\frac{[A-]}{[HA]} \\\\7,4-7,2 = log\frac{[A-]}{[HA]}](/tpl/images/0517/0808/ec2cb.png)
![0,2=log\frac{[A-]}{[HA]} \\\\10^{0,2}=10^{log\frac{[A-]}{[HA]} }\\ \\1,585=\frac{[A-]}{[HA]}](/tpl/images/0517/0808/f0f94.png)
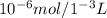
![pH=7,2+log\frac{[0,0613 - 0,01]}{[0,0387 + 0,01]} \\pH=7,22](/tpl/images/0517/0808/d2a0e.png)


