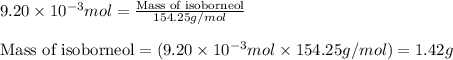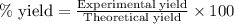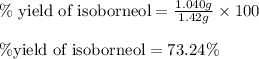Chemistry, 20.02.2020 08:02 tahjaybenloss16
Consider the sodium borohydride reduction of camphor to isoborneol. A reaction was performed in which 1.400 1.400 g of camphor was reduced by an excess of sodium borohydride to make 1.040 1.040 g of isoborneol. Calculate the theoretical yield and percent yield for this reaction.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
You know the right answer?
Consider the sodium borohydride reduction of camphor to isoborneol. A reaction was performed in whic...
Questions


Computers and Technology, 27.05.2021 14:00





SAT, 27.05.2021 14:00


History, 27.05.2021 14:00





Health, 27.05.2021 14:00

Chemistry, 27.05.2021 14:00

Mathematics, 27.05.2021 14:00

Computers and Technology, 27.05.2021 14:00



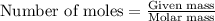 .....(1)
.....(1)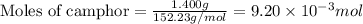
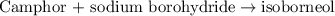
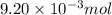 of camphor will produce =
of camphor will produce = 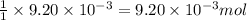 of isoborneol
of isoborneol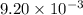 moles
moles