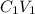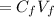Chemistry, 20.02.2020 08:17 Wolfgirl2032
A biochemist carefully measures the molarity of magnesium ion in 47, mL of cell growth medium to be 97. ??. Unfortunately, a careless graduate student forgets to cover the container of growth medium and a substantial amount of the solvent evaporates. The volume of the cell growth medium falls to 6.0 mL. Calculate the new molarity of magnesium ion in the cell growth medium Be sure your answer has the correct number of significant digits. HM x 10

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1
You know the right answer?
A biochemist carefully measures the molarity of magnesium ion in 47, mL of cell growth medium to be...
Questions

Mathematics, 21.06.2020 03:57








Mathematics, 21.06.2020 03:57

Mathematics, 21.06.2020 03:57






Mathematics, 21.06.2020 03:57


Mathematics, 21.06.2020 03:57

Mathematics, 21.06.2020 03:57