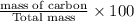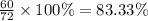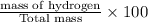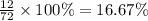Chemistry, 20.02.2020 08:29 ARAYAMYHAND
A hydrocarbon is a compound that contains mostly carbon and hydrogen. Calculate the percent composition (by mass) of the following hydrocarbon: C5H12. Enter the percentages of carbon and hydrogen numerically to four significant figures, separated by commas. View Available Hint(s)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2

Chemistry, 23.06.2019 08:30
Explain how to convert from one unit to another in the metric system.
Answers: 3

Chemistry, 23.06.2019 11:40
Which of the following would have the lowest average kinetic energy
Answers: 1
You know the right answer?
A hydrocarbon is a compound that contains mostly carbon and hydrogen. Calculate the percent composit...
Questions



History, 02.09.2020 06:01

Computers and Technology, 02.09.2020 06:01





Mathematics, 02.09.2020 06:01

History, 02.09.2020 06:01


Mathematics, 02.09.2020 06:01

History, 02.09.2020 06:01

English, 02.09.2020 06:01




History, 02.09.2020 06:01

Mathematics, 02.09.2020 06:01

Biology, 02.09.2020 06:01

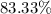 and
and 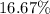 respectively.
respectively.