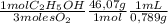Chemistry, 20.02.2020 08:36 ammullims822
The combustion of fuel in your car engine requires oxygen gas, which is supplied as air (21% oxygen molecules) into the engine. Consider a car that is using 100% ethanol, C2H5OH, as fuel. If your engine intakes 3.72 L of air per minute at 1.00 atm and 25ºC, what is the maximum volume of ethanol (0.789 g/mL) that can be burned per minute?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1
You know the right answer?
The combustion of fuel in your car engine requires oxygen gas, which is supplied as air (21% oxygen...
Questions



Mathematics, 02.06.2020 23:00

History, 02.06.2020 23:00

Biology, 02.06.2020 23:00

Physics, 02.06.2020 23:00




Biology, 02.06.2020 23:00


Social Studies, 02.06.2020 23:00

Computers and Technology, 02.06.2020 23:00

Computers and Technology, 02.06.2020 23:00

Mathematics, 02.06.2020 23:00

English, 02.06.2020 23:00

Biology, 02.06.2020 23:00