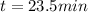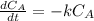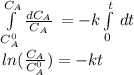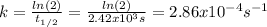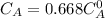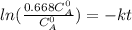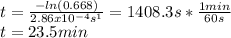Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1
You know the right answer?
The decomposition reaction of A to B is a first-order reaction with a half-life of 2.42×103 seconds:...
Questions




History, 23.10.2021 18:20




Mathematics, 23.10.2021 18:20


Biology, 23.10.2021 18:20


Chemistry, 23.10.2021 18:20



Chemistry, 23.10.2021 18:20

Mathematics, 23.10.2021 18:20


Social Studies, 23.10.2021 18:20


Arts, 23.10.2021 18:20