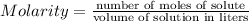Chemistry, 20.02.2020 22:27 mbrisen7420
You make 1.000 L of an aqueous solution that contains 35.0 g of ribose (C5H10O5). How many liters of water would you have to add to this solution to reduce the molarity you calculated in Part A (.233 moles) by a factor of two?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 23.06.2019 13:30
Asap a 50.0 ml soap bubble is blown in a 27.0°c room. it drifts out an open window and lands in a snow bank at -3.0°c. what is its new volume?
Answers: 1
You know the right answer?
You make 1.000 L of an aqueous solution that contains 35.0 g of ribose (C5H10O5). How many liters of...
Questions


Mathematics, 18.03.2021 20:20



Mathematics, 18.03.2021 20:20

Biology, 18.03.2021 20:20

Mathematics, 18.03.2021 20:20


Mathematics, 18.03.2021 20:20

Mathematics, 18.03.2021 20:20


English, 18.03.2021 20:20

Mathematics, 18.03.2021 20:20



Mathematics, 18.03.2021 20:20