Chemistry, 20.02.2020 23:03 NicoleParker
An imaginary element with BCC structure and has an atomic radius of 0.17 nm, with a molar mass of 56.08 g/mol. What is the density of this element in g/cc? hint: you will need Avogadro's number and you will need to convert the given radius to cm.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2
You know the right answer?
An imaginary element with BCC structure and has an atomic radius of 0.17 nm, with a molar mass of 56...
Questions




English, 02.10.2021 14:00




History, 02.10.2021 14:00








Social Studies, 02.10.2021 14:00

English, 02.10.2021 14:00


Mathematics, 02.10.2021 14:00

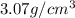
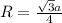
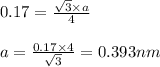
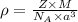
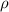 = density
= density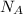 = Avogadro's number =
= Avogadro's number = 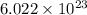
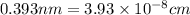 (Conversion factor:
(Conversion factor: 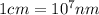 )
)