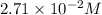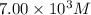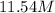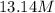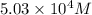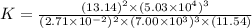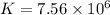Chemistry, 20.02.2020 23:42 corrineikerd
For this heterogeneous system 2A(aq)+3B(g)+C(l) ↽− −⇀ 2D(s)+3E(g) 2A(aq)+3B(g)+C(l)↽−−⇀2D(s)+3E(g) the concentrations and pressures at equilibrium are [A]=2.71× 10 −2 M [A]=2.71×10−2 M , P B =7.00× 10 3 Pa PB=7.00×103 Pa , [C]=11.54 M [C]=11.54 M , [D]=13.14 M [D]=13.14 M , and P E =5.03× 10 4 torr PE=5.03×104 torr . Calculate the thermodynamic equilibrium constant, K K .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1


Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1
You know the right answer?
For this heterogeneous system 2A(aq)+3B(g)+C(l) ↽− −⇀ 2D(s)+3E(g) 2A(aq)+3B(g)+C(l)↽−−⇀2D(s)+3E(g) t...
Questions

Mathematics, 04.05.2021 07:10

English, 04.05.2021 07:10


English, 04.05.2021 07:10



Chemistry, 04.05.2021 07:10



Mathematics, 04.05.2021 07:10


Health, 04.05.2021 07:10






History, 04.05.2021 07:10


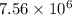
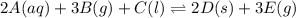
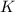 will be,
will be,![K=\frac{[D]^2[E]^3}{[A]^2[B]^3[C]}](/tpl/images/0518/2733/ab45f.png)