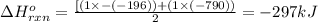Chemistry, 20.02.2020 23:42 xxleeciexx
Use the ΔHrxn values of the following reactions: 2SO2(g) + O2(g) → 2SO3(g) ΔHrxn = –196 kJ 2S(s) + 3O2(g) → 2SO3(g) ΔHrxn = –790 kJ to calculate the ΔHrxn value of this reaction: S(s) + O2(g) → SO2(g) ΔHrxn = ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1
You know the right answer?
Use the ΔHrxn values of the following reactions: 2SO2(g) + O2(g) → 2SO3(g) ΔHrxn = –196 kJ 2S(s) + 3...
Questions



Mathematics, 28.05.2021 02:00

Mathematics, 28.05.2021 02:00

Chemistry, 28.05.2021 02:00

Mathematics, 28.05.2021 02:00

Mathematics, 28.05.2021 02:00


Mathematics, 28.05.2021 02:00


Mathematics, 28.05.2021 02:00


English, 28.05.2021 02:00

Chemistry, 28.05.2021 02:00






Mathematics, 28.05.2021 02:10

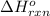 for the reaction is -297 kJ.
for the reaction is -297 kJ.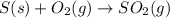
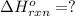
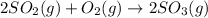
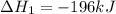
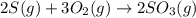
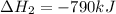
![\Delta H^o_{rxn}=\frac{[1\times (-\Delta H_1)]+[1\times \Delta H_2]}{2}](/tpl/images/0518/2732/98145.png)