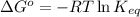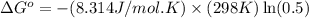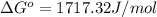Chemistry, 20.02.2020 23:53 DEJAHHARRIS6055
In a unimolecular reaction with twice as much starting material as product at equilibrium, what is the value of Keq? Is ΔG o positive or negative? Enter Keq as a decimal. Be sure to answer all parts.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1
You know the right answer?
In a unimolecular reaction with twice as much starting material as product at equilibrium, what is t...
Questions


Mathematics, 04.03.2021 18:10

Chemistry, 04.03.2021 18:10


Mathematics, 04.03.2021 18:10



Social Studies, 04.03.2021 18:10


Mathematics, 04.03.2021 18:10


Spanish, 04.03.2021 18:10


Biology, 04.03.2021 18:10


Mathematics, 04.03.2021 18:10

Arts, 04.03.2021 18:10

Mathematics, 04.03.2021 18:10

Mathematics, 04.03.2021 18:10

Mathematics, 04.03.2021 18:10

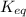 is, 0.5 and
is, 0.5 and 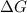 = positive.
= positive.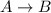
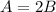 .........(1)
.........(1)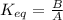 ...........(2)
...........(2)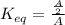
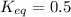
 at 298 K.
at 298 K.