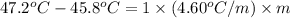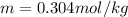Chemistry, 21.02.2020 00:31 athenajames1221
G A student enters the lab and determines the freezing point of pure liquid to be 47.2 ºC. A nonelectrolyte unknown substance is added to the liquid, and the freezing point of the solution is determined to be 45.8 ºC. If the freezing point depression constant for the solvent is 4.60 ºC/molal, what is the molality of the solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

You know the right answer?
G A student enters the lab and determines the freezing point of pure liquid to be 47.2 ºC. A nonelec...
Questions

Chemistry, 15.01.2021 02:20



Biology, 15.01.2021 02:20

Arts, 15.01.2021 02:20


History, 15.01.2021 02:20


Mathematics, 15.01.2021 02:20


History, 15.01.2021 02:20

Mathematics, 15.01.2021 02:20

Biology, 15.01.2021 02:20

Mathematics, 15.01.2021 02:20


Health, 15.01.2021 02:20

Mathematics, 15.01.2021 02:20



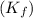 for solvent =
for solvent = 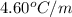
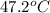
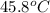
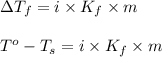
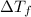 = change in freezing point
= change in freezing point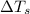 = freezing point of solution =
= freezing point of solution = 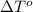 = freezing point of pure liquid =
= freezing point of pure liquid = 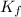 = freezing point constant for solvent =
= freezing point constant for solvent =