
Chemistry, 21.02.2020 01:05 lucifer6669
Determine the moles of H+ reacting with the metal based on the following experimental data. 10.00 mL of 1.00 M HCl solution is added to a sample of Mg metal. The reaction goes to completion and all of the Mg metal is gone. 27.08 mL of 0.30 M NaOH solution is required to reach the end point when titrating the remaining HCl.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1


Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is a physical change? a.burning a piece of wood b.sawing a piece of wood in half c.rust forming on an iron fence d.a copper roof changing color from orange to green
Answers: 1
You know the right answer?
Determine the moles of H+ reacting with the metal based on the following experimental data. 10.00 mL...
Questions





Mathematics, 28.09.2019 00:40

Mathematics, 28.09.2019 00:40

Social Studies, 28.09.2019 00:40


English, 28.09.2019 00:40

Mathematics, 28.09.2019 00:40






Mathematics, 28.09.2019 00:40

World Languages, 28.09.2019 00:40

English, 28.09.2019 00:40


Chemistry, 28.09.2019 00:40

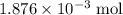 .
.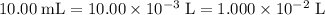 .
.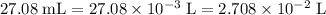 .
.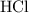 initially present (in the
initially present (in the 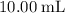 solution at
solution at 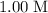 .)
.)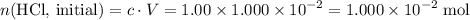 .
. from the titration:
from the titration: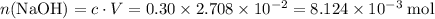 .
.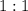 ratio:
ratio: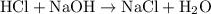 .
.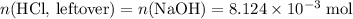 .
.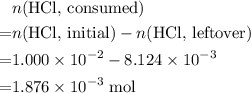 .
.

