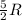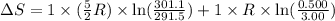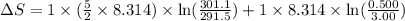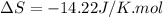Chemistry, 21.02.2020 01:05 AsiaDeas4078
During the test of an internal combustion engine, 3.00 L of nitrogen gas at 18.5 °C was compressed suddenly (and irrevers- ibly) to 0.500 L by driving in a piston. In the process, the tempera- ture of the gas increased to 28.1°C. Assume ideal behavior, what is the change in entropy of the gas?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

You know the right answer?
During the test of an internal combustion engine, 3.00 L of nitrogen gas at 18.5 °C was compressed s...
Questions


Spanish, 28.09.2019 22:20

History, 28.09.2019 22:20

English, 28.09.2019 22:20


Health, 28.09.2019 22:20




Mathematics, 28.09.2019 22:20





Arts, 28.09.2019 22:20



Mathematics, 28.09.2019 22:20

Social Studies, 28.09.2019 22:20

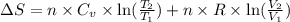
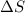 = change in molar entropy
= change in molar entropy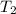 = final temperature =
= final temperature = 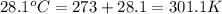
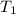 = initial temperature =
= initial temperature = 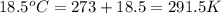
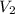 = final volume = 0.500 L
= final volume = 0.500 L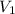 = initial volume = 3.00 L
= initial volume = 3.00 L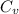 = heat capacity diatomic gas
= heat capacity diatomic gas 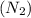 =
=