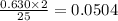Chemistry, 21.02.2020 01:24 INEEDTOPASSS
Determine the balanced chemical equation for this reaction. C8H18(g)+O2(g)→CO2(g)+H2O(g)
Part A:
Enter the coefficients for each compound in order, separated by commas. For example, 1,2,3,4 would indicate one mole of C8H18, two moles of O2, three moles of CO2, and four moles of H2O.
2,25,16,18
Part B:
0.280 mol of octane is allowed to react with 0.630 mol of oxygen. Which is the limiting reactant?
Oxygen
Part C:
How many moles of water are produced in this reaction?
H2O produced = 0.454 mol
Part D:
After the reaction, how much octane is left?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1
You know the right answer?
Determine the balanced chemical equation for this reaction. C8H18(g)+O2(g)→CO2(g)+H2O(g)
...
...
Questions






Social Studies, 16.11.2020 19:30


Mathematics, 16.11.2020 19:30

Computers and Technology, 16.11.2020 19:30

Mathematics, 16.11.2020 19:30


Geography, 16.11.2020 19:30



Biology, 16.11.2020 19:30

Health, 16.11.2020 19:30

Engineering, 16.11.2020 19:30

Mathematics, 16.11.2020 19:30


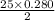 moles of oxygen
moles of oxygen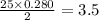
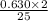 moles of octane will react with the 0.630 mole of oxygen present
moles of octane will react with the 0.630 mole of oxygen present