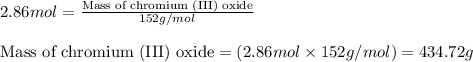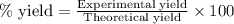Chromium(III) oxide can be prepared by heating chromium(IV) oxide in vacuo at high temperature: 4Cr02 —2Cr2O3 +02 The reaction of 480.1 g of CrO2 yields 402.4 g of Cr203. Calculate the theoretical yield of Cr203 (assuming complete reaction) and its percentage yield. Theoretical yield = Percentage yield = Submit Answer 2 question attempts remaining

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3
You know the right answer?
Chromium(III) oxide can be prepared by heating chromium(IV) oxide in vacuo at high temperature: 4Cr0...
Questions


English, 04.10.2021 14:00

Mathematics, 04.10.2021 14:00

Chemistry, 04.10.2021 14:00

History, 04.10.2021 14:00



Mathematics, 04.10.2021 14:00

Health, 04.10.2021 14:00


Mathematics, 04.10.2021 14:00



Mathematics, 04.10.2021 14:00

Mathematics, 04.10.2021 14:00



Spanish, 04.10.2021 14:00


Mathematics, 04.10.2021 14:00

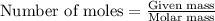 .....(1)
.....(1) = 480.1 g
= 480.1 g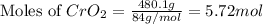
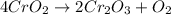
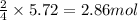 of chromium (III) oxide
of chromium (III) oxide