Chemistry, 21.02.2020 02:32 smusisca53
The change in internal energy for the combustion of 1.0 mol of octane at a pressure of 1.0 atm is -5084.1 kJ. You may want to reference (Pages 381 - 385) Section 9.6 while completing this problem.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1
You know the right answer?
The change in internal energy for the combustion of 1.0 mol of octane at a pressure of 1.0 atm is -5...
Questions

Mathematics, 20.04.2020 23:29

Health, 20.04.2020 23:29


English, 20.04.2020 23:29

Mathematics, 20.04.2020 23:29








Mathematics, 20.04.2020 23:29








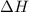 = change in enthalpy = -5074.2 kJ
= change in enthalpy = -5074.2 kJ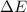 = change in internal energy = -5084.1 kJ
= change in internal energy = -5084.1 kJ