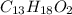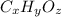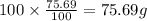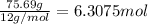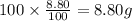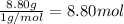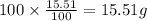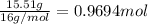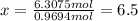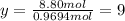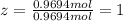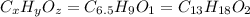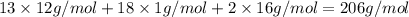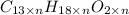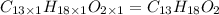Chemistry, 21.02.2020 02:26 sergun252005
Determine the empirical and molecular formulas of each of the following substances. For example, butane has an empirical formula of C2H5 (lowest whole-number ratio) and a molecular formula of C4H10, where the molecular formula corresponds to the molar mass of 58.12 g/mol.
Ibuprofen, a headache remedy, contains 75.69% C, 8.80% H, and 15.51% O by mass and has a molar mass of 206 g/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 23.06.2019 09:30
Large crystals are formed when igneous rocks cool very slowly igneous rocks cool very quickly sedimentary rock is eroded metamorphic rocks change into igneous rock
Answers: 1
You know the right answer?
Determine the empirical and molecular formulas of each of the following substances. For example, but...
Questions

Social Studies, 18.05.2020 22:58


English, 18.05.2020 22:58

Arts, 18.05.2020 22:58





Mathematics, 18.05.2020 22:58










Mathematics, 18.05.2020 22:58

Mathematics, 18.05.2020 22:58