
Chemistry, 21.02.2020 03:00 darenl4478
The Fe 2 + ( 55.845 g/mol) content of a 2.349 g steel sample dissolved in 50.00 mL of an acidic solution was determined by tiration with a standardized 0.100 M potassium permanganate ( KMnO 4 , 158.034 g/mol) solution. The titration required 43.16 mL to reach the end point. What is the concentration of iron in the steel sample? Express your answer as grams of Fe per gram of steel. MnO − 4 + 8 H + + 5 Fe 2 + − ⇀ ↽ − Mn 2 + + 5 Fe 3 + + 4 H 2 O

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1


Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1
You know the right answer?
The Fe 2 + ( 55.845 g/mol) content of a 2.349 g steel sample dissolved in 50.00 mL of an acidic solu...
Questions


History, 02.03.2020 19:32









English, 02.03.2020 19:32



Social Studies, 02.03.2020 19:40

Mathematics, 02.03.2020 19:40

English, 02.03.2020 19:40




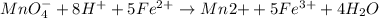
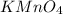 solution = 0.100 M
solution = 0.100 M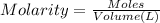
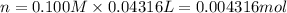
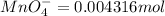
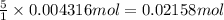 of ferrous ions
of ferrous ions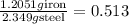 iron per gram of steel
iron per gram of steel

