Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1
You know the right answer?
A 63 gram piece of magnesium absorbs 6689 joules of heat. The final temperature is 663 degrees Celsi...
Questions


Mathematics, 20.02.2020 17:04



Mathematics, 20.02.2020 17:05



Chemistry, 20.02.2020 17:05




Computers and Technology, 20.02.2020 17:05



Mathematics, 20.02.2020 17:06





History, 20.02.2020 17:07

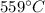
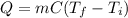
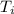 is the initial temperature
is the initial temperature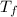 is the final temperature
is the final temperature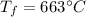 is the final temperature
is the final temperature