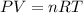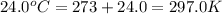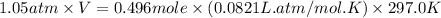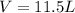Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2


Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1
You know the right answer?
Ammonium carbonate decomposes upon heating according to the following balanced equation:
(NH4)...
(NH4)...
Questions

Chemistry, 13.01.2021 21:20

Arts, 13.01.2021 21:20

Mathematics, 13.01.2021 21:20



Mathematics, 13.01.2021 21:20

English, 13.01.2021 21:20








Mathematics, 13.01.2021 21:20

Mathematics, 13.01.2021 21:20

Mathematics, 13.01.2021 21:20

Mathematics, 13.01.2021 21:20

Mathematics, 13.01.2021 21:20

Arts, 13.01.2021 21:20

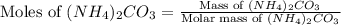
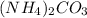 = 96.094 g/mol
= 96.094 g/mol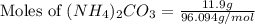
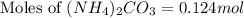
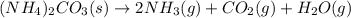
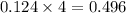 mole of gas
mole of gas