
Chemistry, 21.02.2020 04:48 algahimnada
A 6.19g sample of PCl5 is placed in an evacuated 2.00L flask and is completely vaporized at 252C. 1. Calculate the pressure in the flask if no chemical reaction were to occur. P=.6406 ATM 2.Actually at 252C the PCl5 is partially dissociated according to the following equation: PCl5<->PCl3+Cl2 (all gass states) The observed pressure is found to be 1.00 ATM. In view of this observation, calculate the partial pressure of PCl5 and PCl3 in the flask at 252C.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
A 6.19g sample of PCl5 is placed in an evacuated 2.00L flask and is completely vaporized at 252C. 1....
Questions

Mathematics, 19.11.2019 21:31

English, 19.11.2019 21:31

Mathematics, 19.11.2019 21:31

Mathematics, 19.11.2019 21:31


English, 19.11.2019 21:31



Biology, 19.11.2019 21:31



Mathematics, 19.11.2019 21:31


History, 19.11.2019 21:31


Biology, 19.11.2019 21:31


Mathematics, 19.11.2019 21:31

Health, 19.11.2019 21:31

English, 19.11.2019 21:31

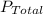 = P₁ + P₂ +...+Pₙ where P₁, P₂, Pₙ are the partial pressures of the constituent gases
= P₁ + P₂ +...+Pₙ where P₁, P₂, Pₙ are the partial pressures of the constituent gases


