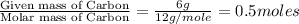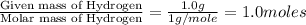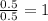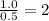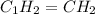Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

You know the right answer?
A 7.0 g sample of a hydrocarbon (a molecule that has only hydrogen and carbon) is subject to combust...
Questions

Mathematics, 10.04.2020 03:15

Mathematics, 10.04.2020 03:15

Mathematics, 10.04.2020 03:15

Mathematics, 10.04.2020 03:15

Law, 10.04.2020 03:15

Geography, 10.04.2020 03:15

Chemistry, 10.04.2020 03:15

Mathematics, 10.04.2020 03:15


Arts, 10.04.2020 03:15

Social Studies, 10.04.2020 03:15

Mathematics, 10.04.2020 03:15



Biology, 10.04.2020 03:15

Mathematics, 10.04.2020 03:15

Mathematics, 10.04.2020 03:15

History, 10.04.2020 03:15


Mathematics, 10.04.2020 03:16

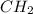
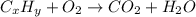
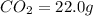
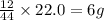 of carbon will be contained.
of carbon will be contained.