
Chemistry, 21.02.2020 05:56 swordnewsnetwork
A 71.0 mL 71.0 mL aliquot of a 1.30 M 1.30 M solution is diluted to a total volume of 248 mL. 248 mL. A 124 mL 124 mL portion of that solution is diluted by adding 133 mL 133 mL of water. What is the final concentration? Assume the volumes are additive.

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2

Chemistry, 23.06.2019 03:30
Scientists often deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each of these numbers in an alternate form.
Answers: 3

Chemistry, 23.06.2019 07:30
Which of the following statements best explains why chemistry is testable a) it can measure data by experiments b) it cannot add new evidence c) it cannot be verified d) it is biased
Answers: 1
You know the right answer?
A 71.0 mL 71.0 mL aliquot of a 1.30 M 1.30 M solution is diluted to a total volume of 248 mL. 248 mL...
Questions

Mathematics, 07.07.2019 08:00

Geography, 07.07.2019 08:00

Social Studies, 07.07.2019 08:00

History, 07.07.2019 08:00

Mathematics, 07.07.2019 08:00



History, 07.07.2019 08:00




World Languages, 07.07.2019 08:00





Biology, 07.07.2019 08:00

Mathematics, 07.07.2019 08:00


Mathematics, 07.07.2019 08:00

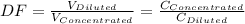
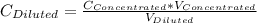 = 1.3×71/248 = 0.372 M
= 1.3×71/248 = 0.372 M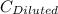 = concentration of new solution
= concentration of new solution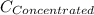 = 0.372 M
= 0.372 M 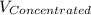 = 124 mL
= 124 mL = 257 mL
= 257 mL

