Chemistry, 21.02.2020 05:58 skylarschumacher7
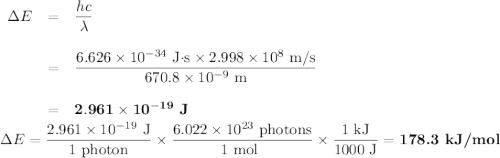
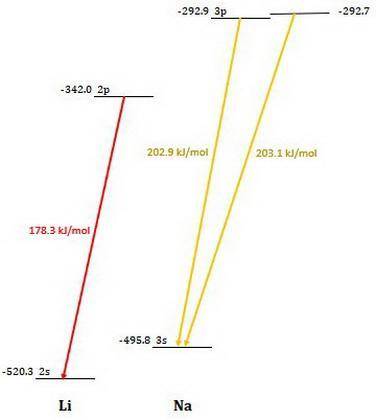
Calculate the photon energy (in kJ/mol) for the single Li emission and the two Na emission wavelengths. To accomplish this, first calculate the energy in units of /photon from Equation (4) and then multiply the result by Avogadro's number to express the energy in /mol of photons. Lastly, divide by 1000 to convert this result to units of kl/mol. Next, use the photon energies to determine the valence orbital energies for both Li and Na. For lithium, the transition is from the 2p- to the 2s-orbital, and the 2s-orbital energy is -520.3 kJ/mol. Use this to find the energy of the 2p-orbitals in Li. For sodium, the higher-energy photon is emitted when the electron drops from one of the 3p-orbitals to the 3s-orbital, while the lower energy photon is emitted when the electron drops from one of the 3d-orbitals to one of the 3p-orbitals. Use these facts, along with the known energy of the 3s-orbital (-495.8 kJ/mol), to find the energies of the 3p- and 3d-orbitals.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3

Chemistry, 23.06.2019 05:30
Astudent made the lewis dot diagram of a compound as shown. mg is written with two dots shown on its top. an o is written on each side of mg. each o has six dots around it. an arrow is shown from one dot on mg toward the vacant space around the o on the right. another arrow is shown from the other dot on mg toward the vacant space around the o on the left. the title of the art is students lewis dot model. what is the error in the lewis dot diagram? an o atom should transfer all its six electrons to mg because the formula is mgo. both electrons of mg should be transferred to one o atom because the formula is mgo. the electrons should be transferred from each o atom to mg because mg has fewer electrons. the number of dots around mg should be four because it has to transfer two electrons to each o.
Answers: 2
You know the right answer?
Calculate the photon energy (in kJ/mol) for the single Li emission and the two Na emission wavelengt...
Questions



Mathematics, 18.12.2020 20:20

Mathematics, 18.12.2020 20:20




Mathematics, 18.12.2020 20:20

Mathematics, 18.12.2020 20:20

Mathematics, 18.12.2020 20:20


Mathematics, 18.12.2020 20:20


Advanced Placement (AP), 18.12.2020 20:20



Mathematics, 18.12.2020 20:20


Mathematics, 18.12.2020 20:20