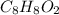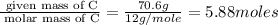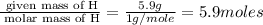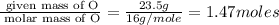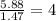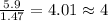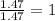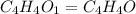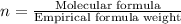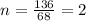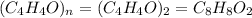Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1

Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1
You know the right answer?
A compound that is composed of carbon, hydrogen, and oxygen contains 70.6% C, 5.9% H, and 23.5% O by...
Questions

Mathematics, 27.10.2020 21:40

Spanish, 27.10.2020 21:40

Mathematics, 27.10.2020 21:40


Mathematics, 27.10.2020 21:40


Physics, 27.10.2020 21:40

Physics, 27.10.2020 21:40

English, 27.10.2020 21:40

Physics, 27.10.2020 21:40


Computers and Technology, 27.10.2020 21:40

Mathematics, 27.10.2020 21:40

English, 27.10.2020 21:40

Computers and Technology, 27.10.2020 21:40

History, 27.10.2020 21:40

Mathematics, 27.10.2020 21:40

Mathematics, 27.10.2020 21:40


Mathematics, 27.10.2020 21:40