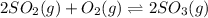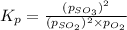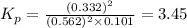Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Figure 10-1 study figure 10-1. the strong nuclear force felt by a single proton in a large nucleus
Answers: 3

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1
You know the right answer?
Consider the reaction below 2SO2(g) + O2(g) ⇌ 2SO3(g) At 1000 K the equilibrium pressures of the thr...
Questions


Mathematics, 01.03.2021 07:10

Mathematics, 01.03.2021 07:20

Mathematics, 01.03.2021 07:20



Mathematics, 01.03.2021 07:20

Engineering, 01.03.2021 07:20

Mathematics, 01.03.2021 07:20

Mathematics, 01.03.2021 07:20

Mathematics, 01.03.2021 07:20


Mathematics, 01.03.2021 07:20

English, 01.03.2021 07:20


Mathematics, 01.03.2021 07:20

Mathematics, 01.03.2021 07:20



English, 01.03.2021 07:20

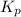 for the reaction is 3.45
for the reaction is 3.45