
Chemistry, 21.02.2020 17:42 macylen3900
Air bags are activated when a severe impact causes a steel ball to compress a spring and electrically ignite a detonator cap. This action causes sodium azide (NaN3) to decompose explosively according to the following reaction. 2 NaN3(s) → 2 Na(s) + 3 N2(g) What mass of NaN3(s) must be reacted to inflate an air bag to 70.6 L at STP?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which traits do human embryos have that link them to a common ancestor with fish and reptiles? a. scales and tail b. gill slits and scales c. tail and gill slits d. hair and tail
Answers: 2


Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1
You know the right answer?
Air bags are activated when a severe impact causes a steel ball to compress a spring and electricall...
Questions



Physics, 27.10.2021 15:50




English, 27.10.2021 15:50

History, 27.10.2021 15:50

Mathematics, 27.10.2021 15:50

Chemistry, 27.10.2021 15:50

Physics, 27.10.2021 15:50

Mathematics, 27.10.2021 15:50



Computers and Technology, 27.10.2021 15:50


Mathematics, 27.10.2021 15:50



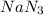 must be reacted to inflate an air bag to 70.6 L at STP.
must be reacted to inflate an air bag to 70.6 L at STP.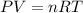
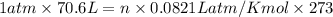
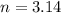
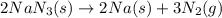
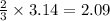 moles of
moles of 

