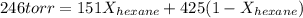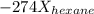Chemistry, 21.02.2020 18:01 lululoveee3433
A solution contains a mixture of pentane and hexane at room temperature. The solution has a vapor pressure of 246 torr . Pure pentane and hexane have vapor pressures of 425 torr and 151 torr, respectively, at room temperature.
a. What is the mole fraction of hexane? (Assume ideal behavior.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1
You know the right answer?
A solution contains a mixture of pentane and hexane at room temperature. The solution has a vapor pr...
Questions

English, 29.11.2019 19:31

Chemistry, 29.11.2019 19:31





Mathematics, 29.11.2019 19:31

History, 29.11.2019 19:31

Biology, 29.11.2019 19:31


Mathematics, 29.11.2019 19:31

Biology, 29.11.2019 19:31



Social Studies, 29.11.2019 19:31

Health, 29.11.2019 19:31


Mathematics, 29.11.2019 19:31


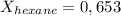
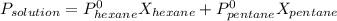 (1)
(1)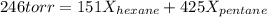 (1)
(1)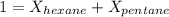 (2)
(2)