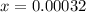Chemistry, 21.02.2020 18:35 michelle8978
Iodine and bromine react to give iodine monobromide, IBr. Ilg) + Br2(g) 2 Br(g) What is the equilibrium composition of a mixture at 145 C that initially contained 0.0019 mol each of iodine and bromine in a 5.0-L vessel? The equilibrium constant K, for this reaction at 145 C is 108.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2

Chemistry, 23.06.2019 10:30
How is it possible for someone to put an ear to a wall and hear someone in the next room? a.sound waves can travel though solids. b.the waves travel from room to room via air. c.there must be some air in the wall so the sound can travel through it. d.sound waves change to electromagnetic waves and then back again.
Answers: 1
You know the right answer?
Iodine and bromine react to give iodine monobromide, IBr. Ilg) + Br2(g) 2 Br(g) What is the equilibr...
Questions

Physics, 07.10.2021 17:10

Biology, 07.10.2021 17:10






Physics, 07.10.2021 17:10


Biology, 07.10.2021 17:10


SAT, 07.10.2021 17:10


Mathematics, 07.10.2021 17:10




Mathematics, 07.10.2021 17:10


Mathematics, 07.10.2021 17:10

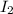 = 0.00006 M
= 0.00006 M 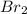 = 0.00006 M
= 0.00006 M 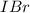 = 0.00064 M
= 0.00064 M 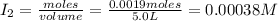
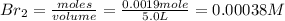
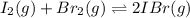
![K_c=\frac{[IBr]^2}{[I_2]\times [Br_2]}](/tpl/images/0519/3166/d47bc.png)
![108=\frac{[2x]^2}{(0.00038-x)^2}](/tpl/images/0519/3166/891f8.png)