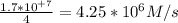Chemistry, 21.02.2020 18:30 naomirice24
The reaction between ethyl bromide (C₂H₅Br) and hydroxide ion in ethyl alcohol at 330 K, , is first order each in ethyl bromide and hydroxide ion. When [C₂H₅Br] is 0.0477 M and [OH⁻] is 0.100 M, the rate of disappearance of ethyl bromide is 1.7×10⁺⁷M/s.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1


Chemistry, 22.06.2019 23:10
Amines are good nucleophiles, even though they are neutral molecules. how would the rate of an sn2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? amines are good nucleophiles, even though they are neutral molecules. how would the rate of an reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? because both reactants in the rate-limiting step are neutral, the reaction will be faster if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will be slower if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will occur at the same rate if the polarity of the solvent is increased. request answer
Answers: 3
You know the right answer?
The reaction between ethyl bromide (C₂H₅Br) and hydroxide ion in ethyl alcohol at 330 K, , is first...
Questions


Biology, 21.04.2021 16:10


Mathematics, 21.04.2021 16:10



Mathematics, 21.04.2021 16:10




Mathematics, 21.04.2021 16:10


Mathematics, 21.04.2021 16:10


English, 21.04.2021 16:10

Mathematics, 21.04.2021 16:10




Mathematics, 21.04.2021 16:10

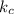 [A] where [A] is the concentration of the reagent
[A] where [A] is the concentration of the reagent![k_{c} \frac{[C_{2} H_{5} Br]}{2} \frac{[OH^{-} ]}{2}](/tpl/images/0519/3086/4c99a.png) =
= 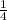 ×Kc[C₂H₅Br][OH⁻]
×Kc[C₂H₅Br][OH⁻]