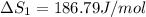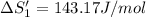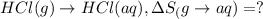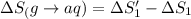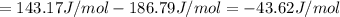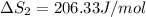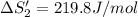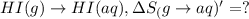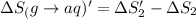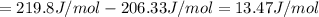All of the hydrogen halides (H-X) are gaseous in their natural state, but dissolve in water to form the aqueous phase.
Entropies (S) for the gaseous H-X molecules (before reaction) are:
HCl (g): 186.79 J/mol
HI (g): 206.33 J/mol
Entropies (S) for the H-X molecules dissolved/solvated in water (after reaction) are:
HCl (aq): 143.17 J/mol
HI (aq): 219.8 J/mol
1. Calculate the ΔS that each of these H-X compounds undergoes as it transitions from the gas phase to the aqueous phase.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1


Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
All of the hydrogen halides (H-X) are gaseous in their natural state, but dissolve in water to form...
Questions


Social Studies, 16.10.2020 06:01

Mathematics, 16.10.2020 06:01

Advanced Placement (AP), 16.10.2020 06:01


English, 16.10.2020 06:01



Mathematics, 16.10.2020 06:01

History, 16.10.2020 06:01

English, 16.10.2020 06:01

English, 16.10.2020 06:01

English, 16.10.2020 06:01

Physics, 16.10.2020 06:01


English, 16.10.2020 06:01




![\Delta S=[\text{Sum of entropy of products}]-[\text{Sum of entropy of reactants}]](/tpl/images/0519/3497/4a681.png)