
Given the equation representing a reaction: H + H ---> H₂What occurs during this reaction? a. A bond is broken and energy is absorbed. b. A bond is broken and energy is released. c. A bond is formed and energy is absorbed. d. A bond is formed and energy is released.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1


Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
Given the equation representing a reaction: H + H ---> H₂What occurs during this reaction? a. A b...
Questions

History, 08.03.2021 04:00

Mathematics, 08.03.2021 04:00



SAT, 08.03.2021 04:00

Mathematics, 08.03.2021 04:00

English, 08.03.2021 04:00

Mathematics, 08.03.2021 04:00

Advanced Placement (AP), 08.03.2021 04:00



Mathematics, 08.03.2021 04:00





Mathematics, 08.03.2021 04:00

Mathematics, 08.03.2021 04:00


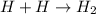 (ΔH = Positive)
(ΔH = Positive)

