
Chemistry, 21.02.2020 21:34 kaoris2322
For each of the following cases, identify the order with respect to the reactant, A. Case (A > product)
1. The half-life of A increases as the initial concentration of A decreases.
2. A twofold increase in the initial concentration of A leads to a fourfold increase in the initial rate.
3.A twofold increase in the initial concentration of A leads to a 1.41-fold increase in the initial rate.
4.The time required for [A] to decrease from [A]0 to [A]0/2 is equal to the time required for [A] to decrease from [A]0/2 to [A]0/4.
5.The rate of decrease of [A] is constant.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 23.06.2019 06:00
Robert leaves a chocolate bar in his car while attending school all day. when he goes to his car in the afternoon, the bat has changed into gooey liquid. what happened to the chocolate bar
Answers: 1
You know the right answer?
For each of the following cases, identify the order with respect to the reactant, A. Case (A > pr...
Questions



History, 06.12.2020 06:30

Mathematics, 06.12.2020 06:30


Arts, 06.12.2020 06:30

Mathematics, 06.12.2020 06:30

Arts, 06.12.2020 06:30

Mathematics, 06.12.2020 06:30

English, 06.12.2020 06:30

Mathematics, 06.12.2020 06:30




Mathematics, 06.12.2020 06:30

Mathematics, 06.12.2020 06:30

Spanish, 06.12.2020 06:30


Computers and Technology, 06.12.2020 06:30

English, 06.12.2020 06:30

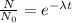
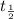 ∝
∝ ![\frac{1}{[A_{0} ]^{n-1} }](/tpl/images/0519/6244/24356.png)


