Chemistry, 21.02.2020 21:53 jalenshayewilliams
5. The gas-phase decomposition of ethyl iodide to give ethylene and hydrogen iodide is a first-order reaction. C2H5I C2H4 + HI At 600 K, the value of k is 1.60 × 10– 5 s– 1. When the temperature is raised to 700 K, the value of k increases to 6.36 × 10– 3 s– 1. What is the activation energy for this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

You know the right answer?
5. The gas-phase decomposition of ethyl iodide to give ethylene and hydrogen iodide is a first-order...
Questions





Physics, 09.03.2021 03:50

English, 09.03.2021 03:50

Mathematics, 09.03.2021 03:50


Mathematics, 09.03.2021 03:50

Mathematics, 09.03.2021 03:50

Mathematics, 09.03.2021 03:50


Mathematics, 09.03.2021 03:50


English, 09.03.2021 03:50



Mathematics, 09.03.2021 03:50

Social Studies, 09.03.2021 03:50

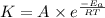
![\log (\frac{K_2}{K_1})=\frac{E_a}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0519/6420/1504e.png)
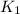 = rate constant at
= rate constant at  =
= 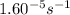
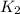 = rate constant at
= rate constant at 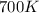 =
= 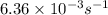
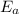 = activation energy for the reaction = ?
= activation energy for the reaction = ?
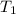 = initial temperature =
= initial temperature = 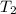 = final temperature =
= final temperature = ![\log (\frac{6.36\times 10^{-3}s^{-1}}{1.60\times 10^{-5}s^{-1}})=\frac{E_a}{2.303\times 8.314J/mole.K}[\frac{1}{600K}-\frac{1}{700K}]](/tpl/images/0519/6420/54c04.png)
![2.60=\frac{E_a}{2.303\times 8.314J/mole.K}[\frac{1}{600K}-\frac{1}{700K}]](/tpl/images/0519/6420/52269.png)