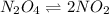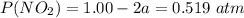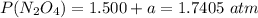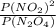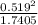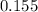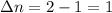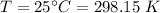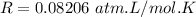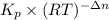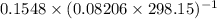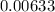Chemistry, 21.02.2020 22:19 hartzpeyton136
A flask is charged with 1.500atm of N2O4(g) and 1.00 atm NO2(g) at 25 degree C, and the following equilibrium is achieved: N2O4(g) 2NO2 After equilibrium is reached, the partial pressure of NO2 is 0.519atm. Calculate the value of Kp for the reaction. Calculate Kc for the reaction.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1
You know the right answer?
A flask is charged with 1.500atm of N2O4(g) and 1.00 atm NO2(g) at 25 degree C, and the following eq...
Questions











Mathematics, 12.07.2021 03:40




English, 12.07.2021 03:40

Physics, 12.07.2021 03:40



Health, 12.07.2021 03:40