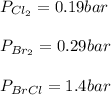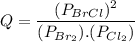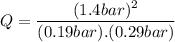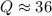Chemistry, 21.02.2020 23:49 Katlynnmarkle13
Consider the gas-phase reaction, Cl2(g) + Br2(g) <=> 2 BrCl(g), for which Kp = 32 at 500 K. If the mixture is analyzed and found to contain 0.19 bar of Cl2, 0.29 bar of Br2 and 1.4 bar of BrCl, describe the situation:a) Q > K and more reactants will be made to reach equilibrium. b) Q > K and more products will be made to reach equilibrium. c) Within 1 decimal place, Q = K and the reaction is at equilibriumd) Q < K and more products will be made to reach equilibrium. e) Q < K and more reactants will be made to reach equilibrium.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
The length of a vector arrow represents its magnitude and the point represents its direction true or false apex
Answers: 3

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1
You know the right answer?
Consider the gas-phase reaction, Cl2(g) + Br2(g) <=> 2 BrCl(g), for which Kp = 32 at 500 K. If...
Questions

Business, 15.12.2020 22:30



English, 15.12.2020 22:30

Mathematics, 15.12.2020 22:30

History, 15.12.2020 22:30

Mathematics, 15.12.2020 22:30

Biology, 15.12.2020 22:30

Mathematics, 15.12.2020 22:30


Spanish, 15.12.2020 22:30

Mathematics, 15.12.2020 22:30



Mathematics, 15.12.2020 22:30

Mathematics, 15.12.2020 22:30