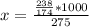Potassium hydrogen phosphate is used in the preparation of non-dairy powdered creamers. Calculate the molarity of a solution prepared by dissolving 238 g of K2HPO4 in enough water to produce 275 mL of solution. A) 0.732 M B) 0.865 M. C) 2.66 M D) 4.97 M E) none of the above

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2
You know the right answer?
Potassium hydrogen phosphate is used in the preparation of non-dairy powdered creamers. Calculate th...
Questions




World Languages, 14.12.2020 22:50

Medicine, 14.12.2020 22:50

History, 14.12.2020 22:50



English, 14.12.2020 22:50

Biology, 14.12.2020 22:50

Mathematics, 14.12.2020 22:50


Mathematics, 14.12.2020 22:50

Mathematics, 14.12.2020 22:50

Mathematics, 14.12.2020 22:50

Mathematics, 14.12.2020 22:50




Biology, 14.12.2020 22:50

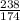 moles.
moles.