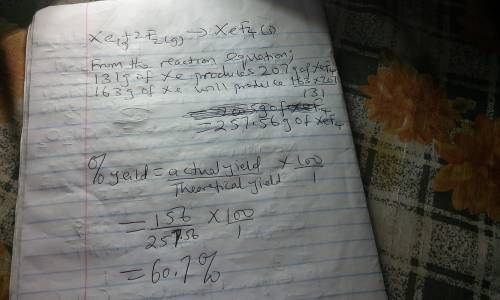Chemistry, 22.02.2020 01:45 cjtambasco
Although they were formerly called the inert gases, at least the heavier elements of Group 8 do form relatively stable compounds. For example, xenon combines directly with elemental fluorine at elevated temperatures in the presence of a nickel catalyst. Use table 1 and table 2. Xe(g) + 2 F2(g) → XeF4(s) What is the theoretical mass of xenon tetrafluoride that should form when 163 g of xenon is reacted with 164 g of F2? g= What is the percent yield if only 156 g of XeF4 is actually isolated?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2


Chemistry, 23.06.2019 07:30
The compound formed from 2 atoms of hydrogen and one atom of oxygen is
Answers: 1

Chemistry, 23.06.2019 10:00
State the effect on the concentration of the clo- ion when there is a decrease in the concentration of the oh- ion
Answers: 1
You know the right answer?
Although they were formerly called the inert gases, at least the heavier elements of Group 8 do form...
Questions

Computers and Technology, 31.03.2020 15:50

Mathematics, 31.03.2020 15:52







English, 31.03.2020 15:57


Advanced Placement (AP), 31.03.2020 15:57


Mathematics, 31.03.2020 15:57

Business, 31.03.2020 15:57

Mathematics, 31.03.2020 15:57




English, 31.03.2020 15:57